Iron (Fe) Uptake in Plants
Plants utilize two separate mechanisms to solubilize the insoluble ferric Fe [Fe(III)] in the rhizosphere and transport it through the plasma membrane (PM):
1) reduction-based strategy (strategy I)
2) chelation-based strategy (strategy II)
1) reduction-based strategy (strategy I)
2) chelation-based strategy (strategy II)
Strategy I: The Reduction Strategy
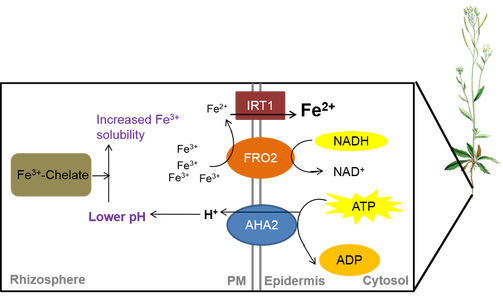
In Strategy I plants, all dicots and non-graminaceous monocots such as Arabidopsis thaliana, Fe acquisition consists of three activities on PM of root epidermal cells:
1) rhizosphere acidification by PM-localized H+-ATPase (AHA)-mediated proton extrusion, 2) reduction of insoluble Fe(III) to soluble ferrous [Fe(II)] by the PM-localized oxidoreductase, named FERRIC CHELATE REDUCTASE (FRO),
3) consequent transport of Fe(II) ion into the root cells by a member of the ZINC (Zn)–Fe-REGULATED TRANSPORTER (ZIP) family of metal transporters.
1) rhizosphere acidification by PM-localized H+-ATPase (AHA)-mediated proton extrusion, 2) reduction of insoluble Fe(III) to soluble ferrous [Fe(II)] by the PM-localized oxidoreductase, named FERRIC CHELATE REDUCTASE (FRO),
3) consequent transport of Fe(II) ion into the root cells by a member of the ZINC (Zn)–Fe-REGULATED TRANSPORTER (ZIP) family of metal transporters.
Strategy II: The Chelation Strategy
In Strategy II plants, which include Graminaceous plants such as wheat, rice and maize, phytosiderophores (PSs) are released into the rhizosphere, where they solubilize Fe(III) by forming a complex with it, and then the Fe(III)-PS complex is taken up into the root cells via the YELLOW STRIPE (YS) family of transporters, named for maize (Zea mays) ZmYS1 transporter.
Similar to Strategy I plants, some grasses can also acquire Fe(II) in addition to Fe(III)-PS by help of a Fe(II) transporter (Itai et al., 2000; Ishimaru et al., 2006).
Similar to Strategy I plants, some grasses can also acquire Fe(II) in addition to Fe(III)-PS by help of a Fe(II) transporter (Itai et al., 2000; Ishimaru et al., 2006).
Iron Distribution
Once Fe(II) is absorbed into the roots, various transporters and chemicals are involved in its inter- and intracellular mobilization for storage and utilization.
Intercellular Fe Distribution
Fe(II) can be chelated by nicotianamine (NA) and transported intercellularly by YELLOW STRIPE-LIKE (YSL) transporters (DiDonato et al., 2004; Waters et al., 2006; Waters and Grusak, 2008; Chu et al., 2010).
Aforementioned ZmYS1 transporter is the first identified protein of the YSL family. It functions in root Fe-PS uptake from the rhizosphere, and it is suggested that YS1 may also be involved in root-to-shoot distribution of Fe since it is upregulated in both roots and shoots under Fe deficiency (Curie et al., 2001). It is strongly believed that the YSL family of proteins transport Fe(II)-NA in both grasses and non-grasses, and Fe(III)-PS specifically in grasses. They function in Fe(III)-PS uptake from the rhizosphere into the roots in grasses, and Fe(II)-NA distribution in graminoids as well as non-graminoids. YSL family belongs to a novel family of OLIGOPEPTIDE TRANSPORTERS (OPTs) and have been shown to function in transit metal movements through the plasma membrane.
In the vasculature, citrate, which is exuded by a FERRIC REDUCTASE DEFECTIVE3 (FRD3) transporter (Durrett et al., 2007), forms a tri-Fe(III), tri-citrate complex, first identified in tomato xylem sap, for long-distance transport (Rogers and Guerinot, 2002; Green and Rogers, 2004; Rellan-Alvarez et al., 2010).
Aforementioned ZmYS1 transporter is the first identified protein of the YSL family. It functions in root Fe-PS uptake from the rhizosphere, and it is suggested that YS1 may also be involved in root-to-shoot distribution of Fe since it is upregulated in both roots and shoots under Fe deficiency (Curie et al., 2001). It is strongly believed that the YSL family of proteins transport Fe(II)-NA in both grasses and non-grasses, and Fe(III)-PS specifically in grasses. They function in Fe(III)-PS uptake from the rhizosphere into the roots in grasses, and Fe(II)-NA distribution in graminoids as well as non-graminoids. YSL family belongs to a novel family of OLIGOPEPTIDE TRANSPORTERS (OPTs) and have been shown to function in transit metal movements through the plasma membrane.
In the vasculature, citrate, which is exuded by a FERRIC REDUCTASE DEFECTIVE3 (FRD3) transporter (Durrett et al., 2007), forms a tri-Fe(III), tri-citrate complex, first identified in tomato xylem sap, for long-distance transport (Rogers and Guerinot, 2002; Green and Rogers, 2004; Rellan-Alvarez et al., 2010).
Subcellular Fe Distribution
Chloroplasts and mitochondria are the major Fe usage sites within plant cells (Nouet et al., 2011). In the form of Fe-S clusters, chloroplast Fe makes up 70–90% of cellular iron in the mesophyll cells (Nouet et al., 2011). Besides chloroplasts and mitochondria, Fe is also found in nucleolus in Arabidopsis leaves and pea embryo (Roschzttardtz et al., 2011).
Vacuole
Fe is mainly accumulated in globoid structures of protein storage vacuoles in endodermal cells of Arabidopsis and wheat embryos (Roschzttardtz et al., 2009). VACUOLAR IRON TRANSPORTER1 (VIT1) is responsible for Fe(II) transport into the vacuole in the Arabidopsis embryo (Kim et al., 2006). In contrast to VIT1, AtNRAMP3 and AtNRAMP4 play a role in Fe export from vacuole to the cytoplasm both during germination and in other developmental stages (Lanquar et al., 2005).
Plastids and Mitochondria
PERMEASE IN CHLOROPLASTS1 (PIC1) is localized to the inner membrane of chloroplasts and functions in Fe transport into the chloroplasts (Duy et al., 2007). Localization of AtFRO7 (FERRIC-CHELATE REDUCTASE OXIDASE7) in the chloroplast envelope suggests the potential role of reduction machinery in Fe translocation into the chloroplasts (Jeong et al., 2008). Additionally, MAR1/IREG3 (MULTIPLE ANTIBIOTIC RESISTANCE1/IRON-REGULATED PROTEIN3) may be involved in Fe-NA complex transport into the plastids because of the sequence similarity to other IREG family of proteins, and the reversible leaf chlorosis of MAR1 overexpression plants by Fe application (Conte et al., 2009; Conte and Lloyd, 2010).
In Arabidopsis, one of the ABC family of proteins, ATP-BINDING CASSETTE TRANSPORTERS OF MITOCHONDRIA3 (ATM3), is suggested to function in the export of Fe-S clusters from mitochondria. Moreover, MITOCHONDRIAL FE TRANSPORTER (MIT)/MITOFERRITIN functions in Fe influx into the mitochondria in rice (Bashir et al., 2011).
YSL isoforms localize to diverse organelles. HvYSL5 is localized to the vesicles or the tonoplast in barley (Zheng et al., 2011); OsYSL6 signals indicate a cytosolic localization in rice (Sasaki et al., 2011); Arabidopsis AtYSL4 and AtYSL6 are localized to the plastids in one study , or to the vacuolar membranes/the internal membranes similar to the endoplasmic reticulum in another study (Conte et al., 2013).
In Arabidopsis, one of the ABC family of proteins, ATP-BINDING CASSETTE TRANSPORTERS OF MITOCHONDRIA3 (ATM3), is suggested to function in the export of Fe-S clusters from mitochondria. Moreover, MITOCHONDRIAL FE TRANSPORTER (MIT)/MITOFERRITIN functions in Fe influx into the mitochondria in rice (Bashir et al., 2011).
YSL isoforms localize to diverse organelles. HvYSL5 is localized to the vesicles or the tonoplast in barley (Zheng et al., 2011); OsYSL6 signals indicate a cytosolic localization in rice (Sasaki et al., 2011); Arabidopsis AtYSL4 and AtYSL6 are localized to the plastids in one study , or to the vacuolar membranes/the internal membranes similar to the endoplasmic reticulum in another study (Conte et al., 2013).
Other Organelles
In addition to Fe transporters found in vacuole, chloroplast and mitochondria, one of the members of MATE family of proteins, BCD1 (BUSH-AND-CHLOROTIC-DWARF1), localizes to the Golgi complex and contributes to iron homoeostasis during stress responses and senescence in Arabidopsis (Seo et al., 2012).